
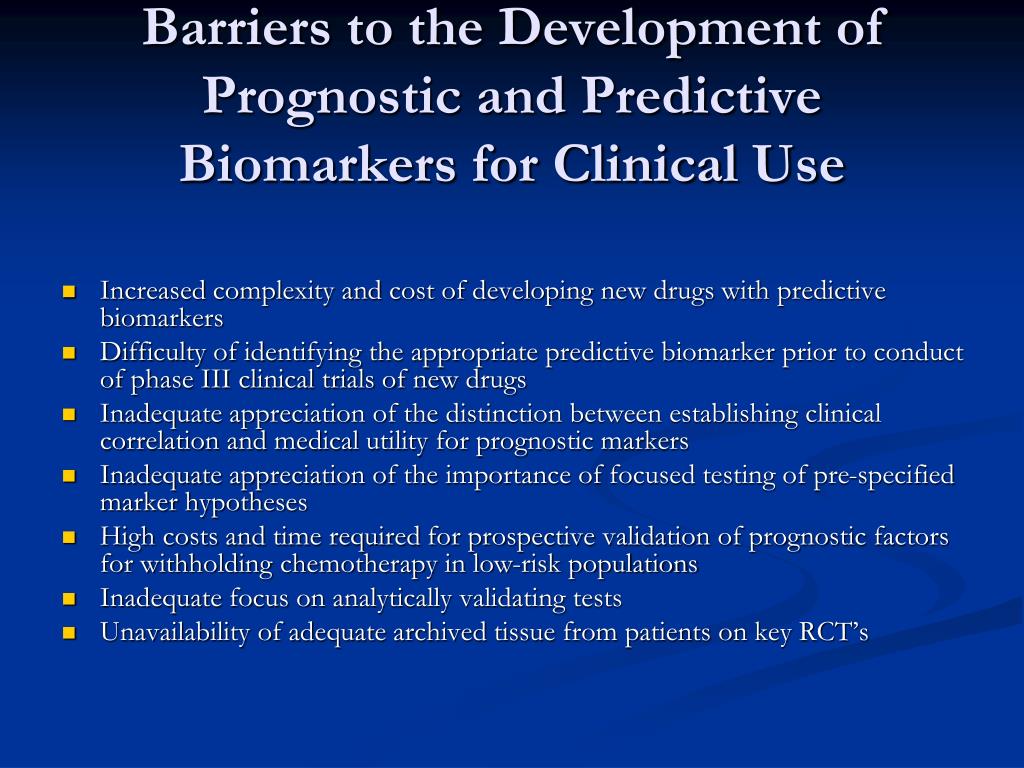
Ĭommon examples of predictive biomarkers are genes such as ER, PR and HER2/neu in breast cancer, BCR-ABL fusion protein in chronic myeloid leukaemia, c-KIT mutations in GIST tumours and EGFR1 mutations in NSCLC. For example, in metastatic colorectal cancer predictive biomarkers can serve as a way of evaluating and improving patient survival rates and in the individual case by case scenario, they can serve as a way of sparing patients from needless toxicity that arises from cancer treatment plans. This offers a dual approach to both seeing trends in retrospective studies and using biomarkers to predict outcomes. For example, molecular biomarkers situated at the interface of pathology-specific molecular process architecture and drug mechanism of action promise capturing aspects allowing assessment of an individual treatment response. Predictive biomarkers are used to help optimize ideal treatments, and often indicate the likelihood of benefiting from a specific therapy. Predictive molecular, cellular, or imaging biomarkers that pass validation can serve as a method of predicting clinical outcomes. All four types of biomarkers have a clinical role in narrowing or guiding treatment decisions and follow a sub-categorization of being either predictive, prognostic, or diagnostic. The four main classes are molecular, physiologic, histologic and radiographic biomarkers. Biomarkers used in the medical field, are a part of a relatively new clinical toolset categorized by their clinical applications.


 0 kommentar(er)
0 kommentar(er)
